Dermatologic Conditions
(Section 1)
Case 5
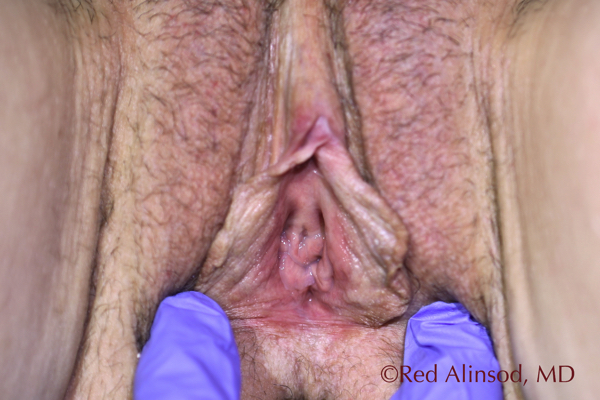 Introitus
Introitus
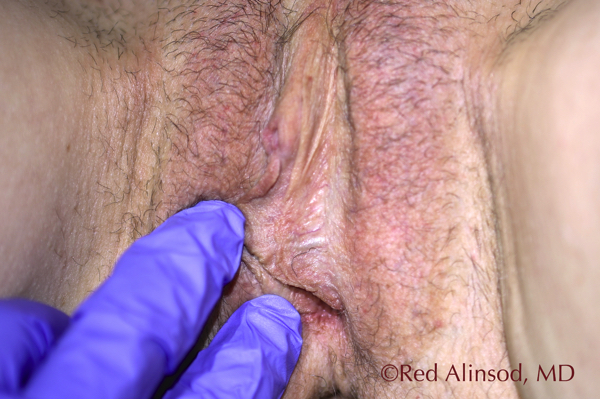 Left Side
Left Side
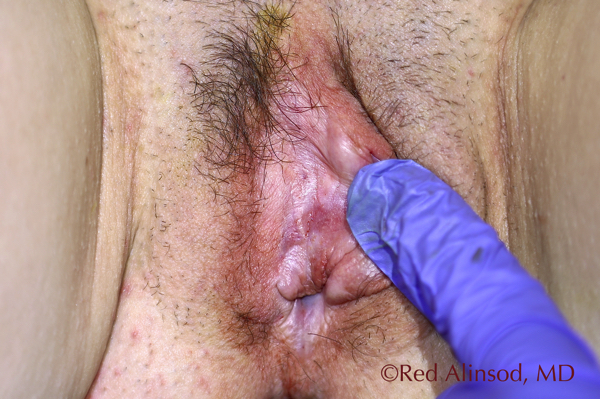 Right Side
Right Side
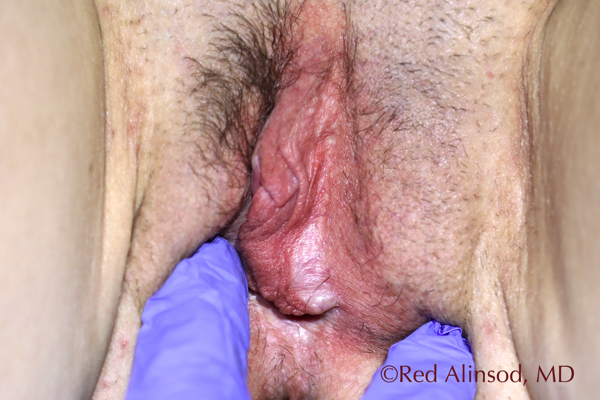 Left Side Introitus
Left Side Introitus
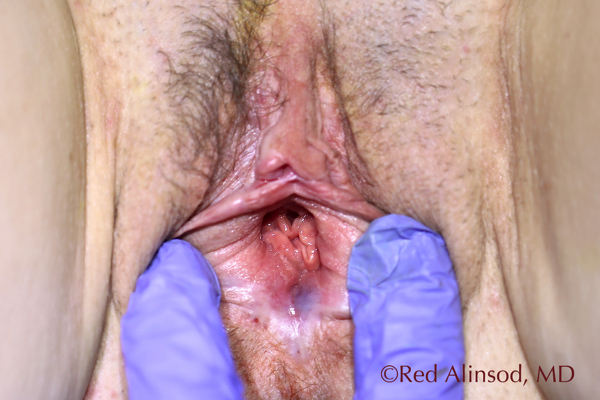 Introitus June 2016
Introitus June 2016
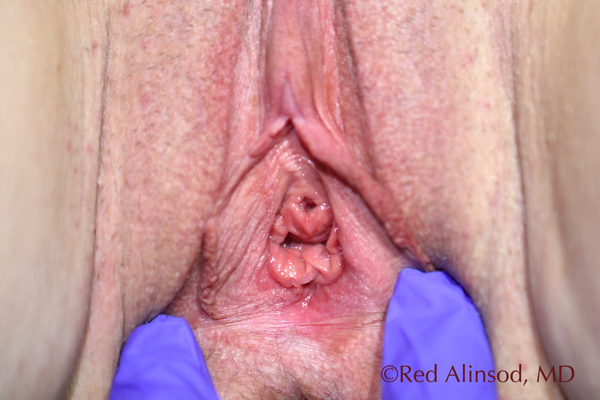 Introitus September 2016
Introitus September 2016
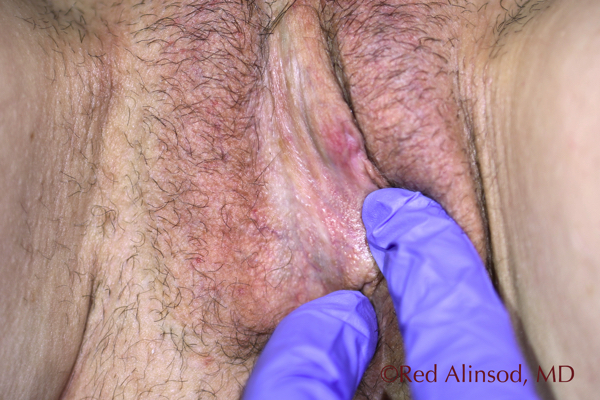 Introitus Right Side November 2016
Introitus Right Side November 2016
 Introitus
Introitus
 Left Side
Left Side
 Right Side
Right Side
 Left Side Introitus
Left Side Introitus
 Introitus June 2016
Introitus June 2016
 Introitus September 2016
Introitus September 2016
 Introitus Right Side November 2016
Introitus Right Side November 2016