Loose Skin
(Section 2)
Case 4
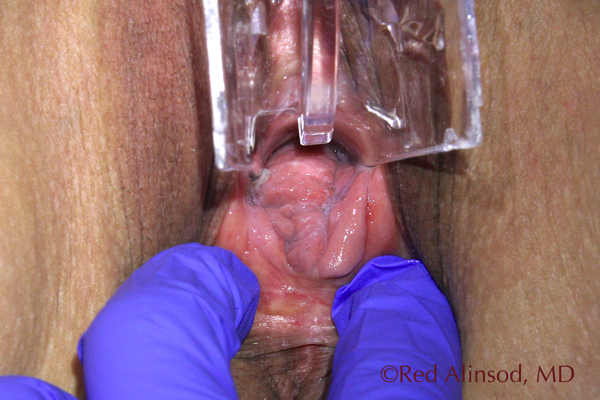 Before ThermiVa Max Valsalva Reduced Rugation
Before ThermiVa Max Valsalva Reduced Rugation
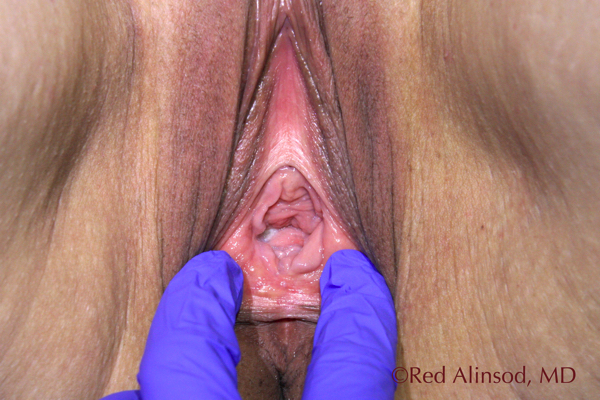 Before ThermiVa at Rest
Before ThermiVa at Rest
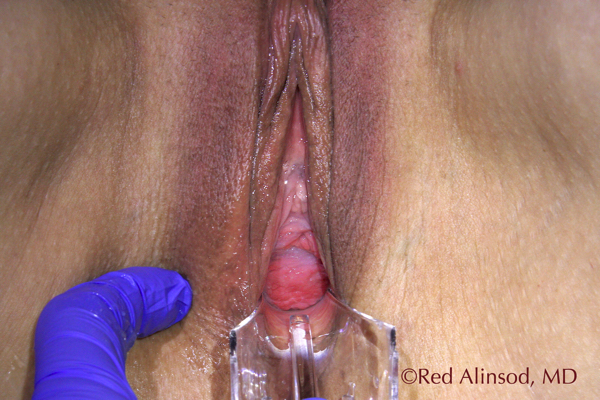 Before ThermiVa Max Valsala
Before ThermiVa Max Valsala
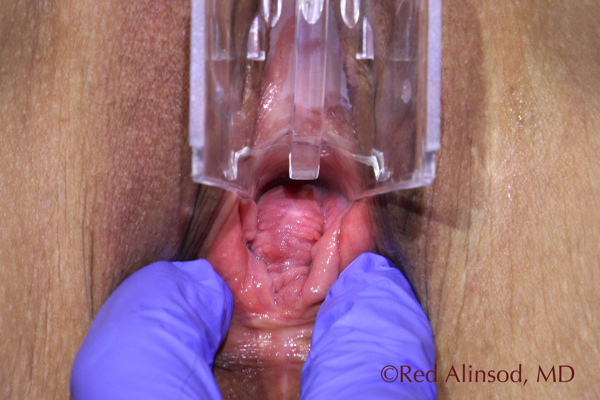 After ThermiVa Max Valsalva
After ThermiVa Max Valsalva
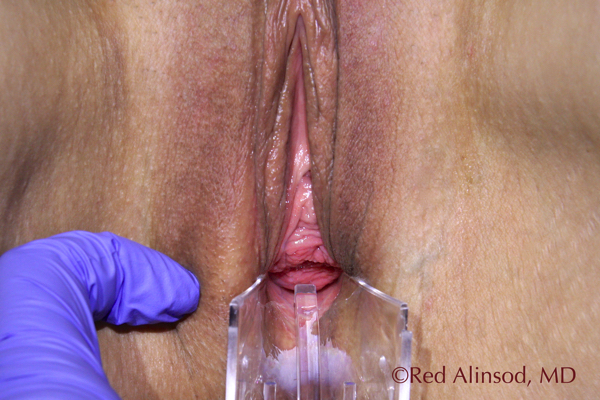 2-Years Post ThermiVa Max Valsalva Rugations
2-Years Post ThermiVa Max Valsalva Rugations
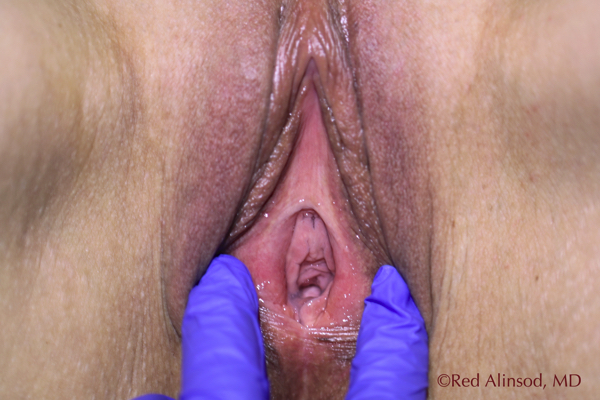 2-Years Post ThermiVa at Rest After Touch-Up
2-Years Post ThermiVa at Rest After Touch-Up
 Before ThermiVa Max Valsalva Reduced Rugation
Before ThermiVa Max Valsalva Reduced Rugation
 Before ThermiVa at Rest
Before ThermiVa at Rest
 Before ThermiVa Max Valsala
Before ThermiVa Max Valsala
 After ThermiVa Max Valsalva
After ThermiVa Max Valsalva
 2-Years Post ThermiVa Max Valsalva Rugations
2-Years Post ThermiVa Max Valsalva Rugations
 2-Years Post ThermiVa at Rest After Touch-Up
2-Years Post ThermiVa at Rest After Touch-Up