Combination Surgery and ThermiVa
Case 7
 Front-Side Pre-Op
Front-Side Pre-Op
 Front-Side Pre-Op
Front-Side Pre-Op
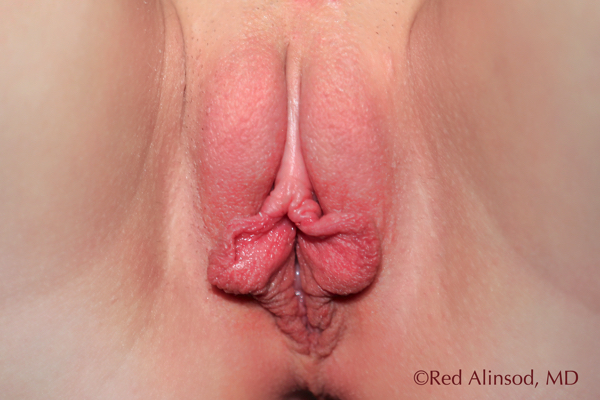 Front Pre-Op
Front Pre-Op
 Rear Pre-Op
Rear Pre-Op
 Side-Lying Pre-Op
Side-Lying Pre-Op
 Front-Side Post-Op
Front-Side Post-Op
 Front-Side Post-Op
Front-Side Post-Op
 Front Post-Op 6-Months
Front Post-Op 6-Months
 Rear-Lying Post-Op
Rear-Lying Post-Op
 Side-Lying Post-Op
Side-Lying Post-Op
 Front-Side Pre-Op
Front-Side Pre-Op
 Front-Side Pre-Op
Front-Side Pre-Op
 Front Pre-Op
Front Pre-Op
 Rear Pre-Op
Rear Pre-Op
 Side-Lying Pre-Op
Side-Lying Pre-Op
 Front-Side Post-Op
Front-Side Post-Op
 Front-Side Post-Op
Front-Side Post-Op
 Front Post-Op 6-Months
Front Post-Op 6-Months
 Rear-Lying Post-Op
Rear-Lying Post-Op
 Side-Lying Post-Op
Side-Lying Post-Op